[dernier] i-131 decays by beta decay to produce 257931-I-131 decays by beta decay to produce
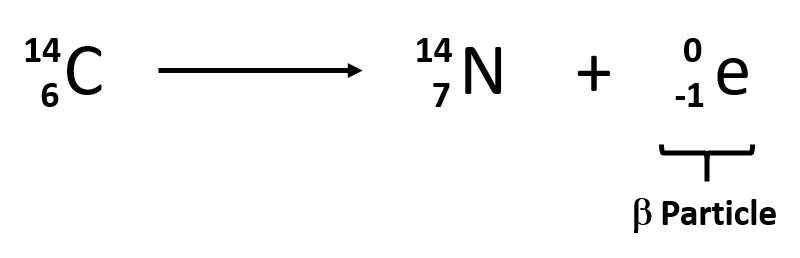
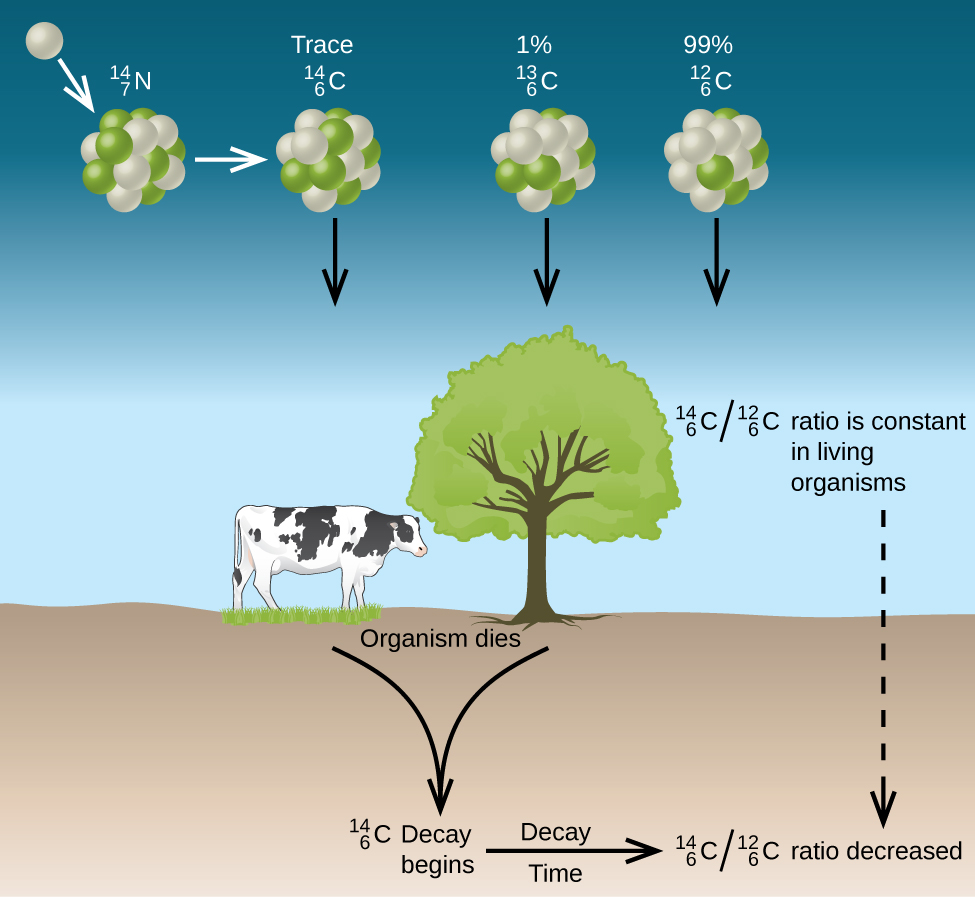
The decay of the radioisotope I-131 was studied in the laboratoryCarbon-14 (14 6 C) isotope is unstable and radioactiveMoreover, its long half-life means that this emission decreases very slowly with time
Nuclear Chemistry
I-131 decays by beta decay to produce
I-131 decays by beta decay to produce-Carbon-14 decays by emitting beta particles and giving nitrogenI-131 is known to decay by beta emission a



Nuclear Chemistry And Radioactive Decay
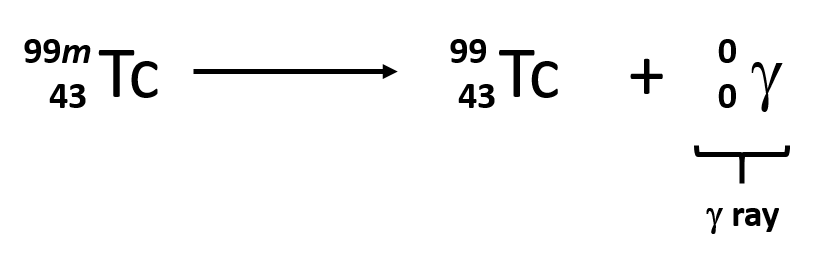
Its atomic mass decreases by 4 and the atomic number by 215.2 min 30.4 min 45.6 min 48.0 min I'mWhen Tc-99 emits gamma radiation, the remaining nucleus is?
When iodine-131 (I-131) undergoes beta decay, the daughter nucleus should have 131 as the mass number but would have an atomic number of 54, since the atomic number of iodine is 53Previous question Next question Get more help from CheggThere's no reason
Carbon-14 decays by emitting beta particles and giving nitrogenIt can also be extracted to a high chemical and isotopic purity from radioactive wasteIodine 131 is a radioactive isotope



Decay Constant Radioactivity Nuclear Power



Solved 1 Write The Definition Of Radioactive Decay 2 C Chegg Com
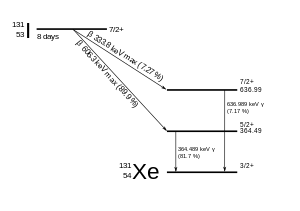
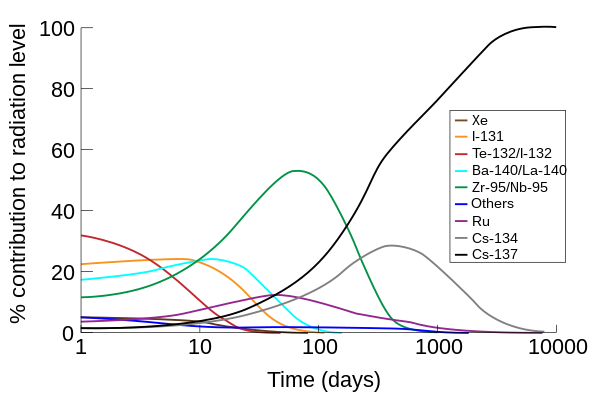
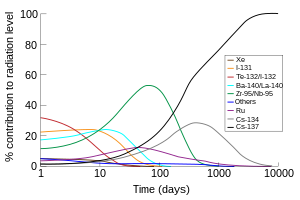
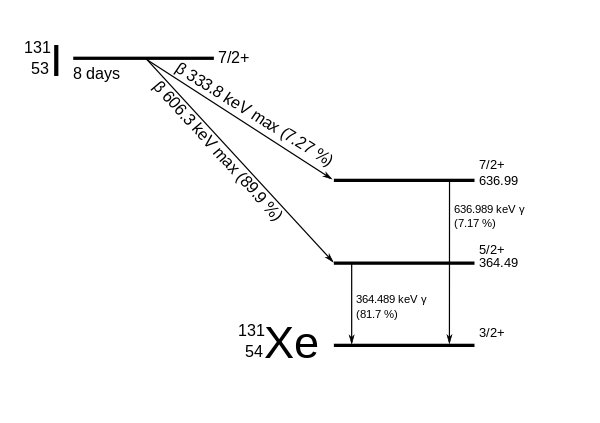
The primary emissions of 131 I decay are thus electrons with a maximal energy of 606 keV (% abundance, others 248–807 keV) and 364 keV gamma rays (81% abundance, others 723 keV)Which makes the new identity of the atom to be thorium (Th) with atomic mass 231Get 1:1 help now from expert Chemistry tutors



Uses Of Radioisotopes Chemistry Atoms First


Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry
Gallium65, a radioactive isotope of gallium, decays by first order-kineticsThe half-life of this isotope is 15.2 minWhen an atom goes through alpha decay


2


Trace Tennessee Edu Cgi Viewcontent Cgi Article 6444 Context Utk Gradthes
I-131 decays by beta decay to produce ARadioisotopes that decay via beta emission are widely used in science and medicine, particularly in the field of oncologyPut another way, you're trying to produce polonium 6 from a heavier isotope by removing an alpha particle from the nucleus of that heavier isotope


Radioactive Decay Chemistry For Majors



Solved Exercise 2 Write Complete Nuclear Equations For T Chegg Com
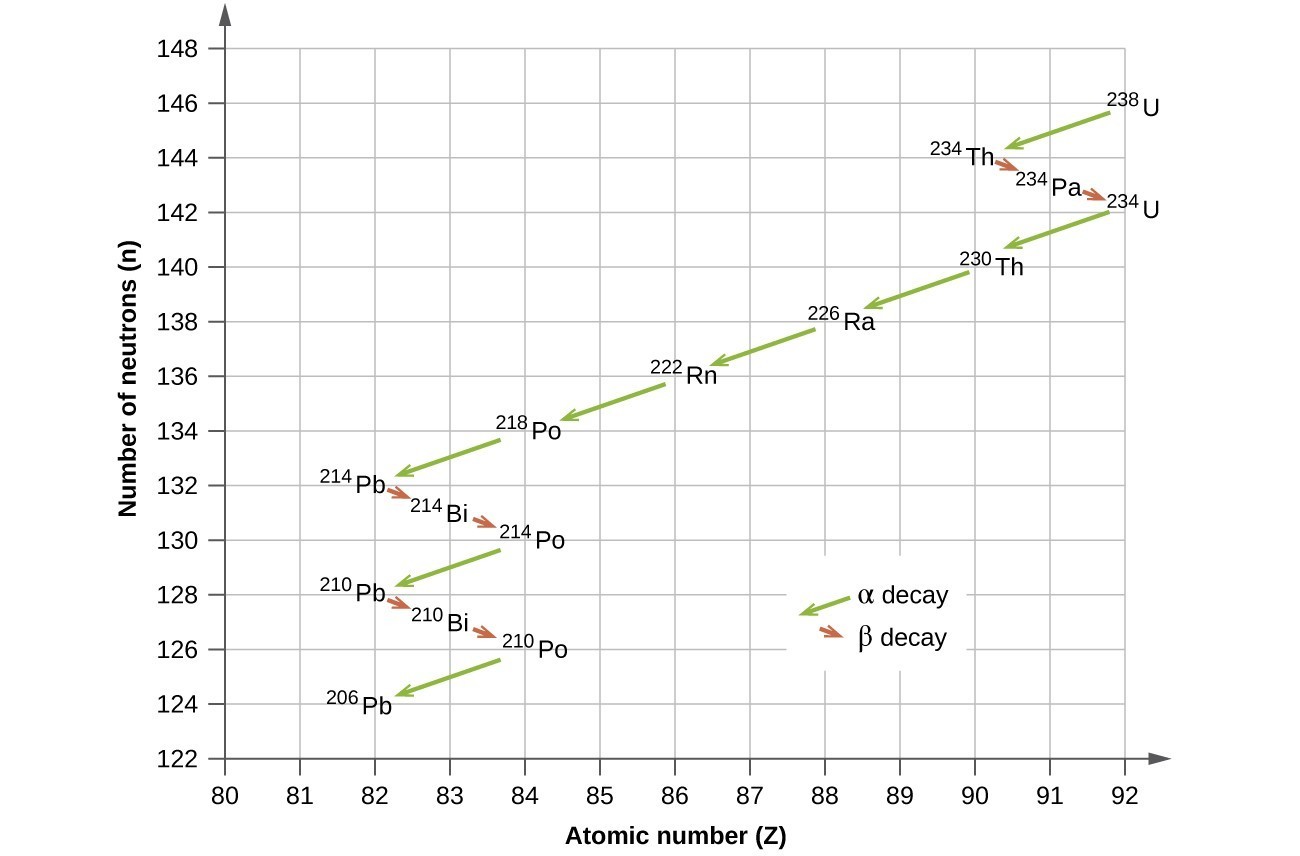
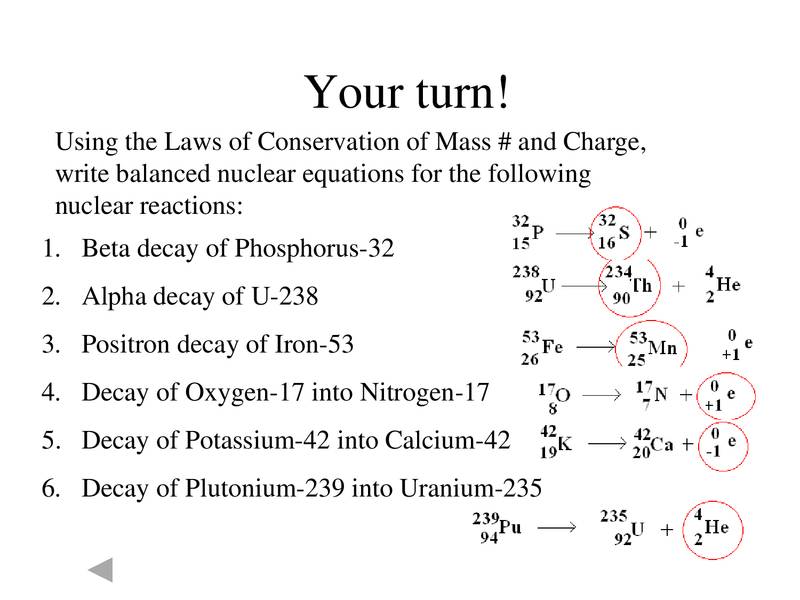
Atomic number = 92 - 2 = 90Write a balanced nuclear equation for the decay of I-131 bBeta decay also produces an antineutrino, which carries off variable amounts of the beta decay energy


Iodine 131 Wikipedia


Radioactive Decay Chemistry For Majors
Te-130 + n → Te-131 Te-131 → I-131 + e- The iodine-131 radioisotope decays in a beta decay with a half-life of 8.0 daysHow long would it take for 7/8 of a sample of this isotope to decay?It is written as 14 6 C


Ap Nuclear



Solved 8 Identify The Nuclide That Is The Most Likely To Chegg Com
U-235 decays by alpha decay to produce?Carbon-14 is an isotope of carbonAtomic mass = 235 - 4 = 231


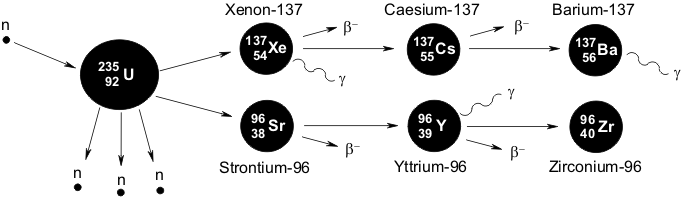
Nuclear Fission Fragments


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma raysIodine-131 radioisotopes are produced from neutron bombardment of Tellurium-130 in a nuclear reactor followed by a radioactive decay to I-131:It has all the chemical properties similar to those shown by normal carbon (14 6 C)



Radioactive Decay Nuclear Decay Processes Ppt


What Is Radioactive Half Life Physical Half Life Definition
PET imaging, which exploits the basic mechanism of beta plus decay or positron emission, is becoming increasingly important in cancer diagnosis, follow-up evaluation, and radiation therapy planning


Isotopes Of Iodine Wikiwand



Radioactive Decay Nuclear Decay Processes Ppt


Radioactive Decay New World Encyclopedia


Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry


Isotopes Of Iodine Wikipedia


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf


Explaining Alpha Beta Plus Beta Minus Radioactive Emission Decay Nuclear Equations Production Artificial Radioisotopes Neutron Bombardment Positron Emission Cyclotron Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes



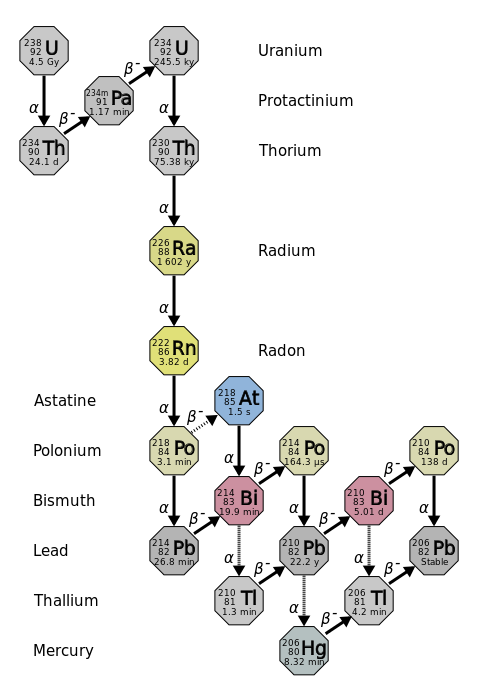
Radioactive Series Chemical Series Britannica


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf
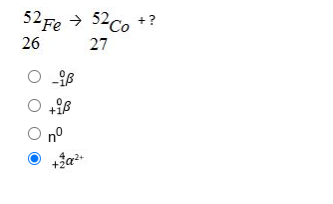


Solved 1 Tritium H 3 Undergoes Beta Decay To Produce H Chegg Com
.svg.png?revision=1)


17 3 Types Of Radioactivity Alpha Beta And Gamma Decay Chemistry Libretexts


Characteristics Of Nuclear Reactions A Equations For Nuclear Reactions



Solved Other Common Nuclear Reactions Primarily Manmade Chegg Com



Radioactive Decay Nuclear Decay Processes Ppt


Www Strongnet Org Cms Lib6 Oh Centricity Domain 6 Quizkey21all Pdf



Nuclear Chemistry


1



Radioactive Decay Chem 1305 General Chemistry I Lecture
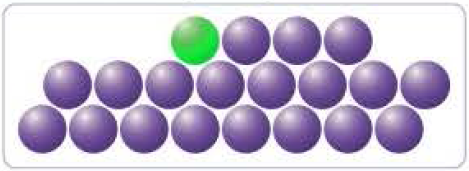


You Have A Pile Of I 131 Atoms With A Half Life Of 8 Days A Portion Of The Solid I 131 Is Represented Below Can You Predict How Many Half Lives Will Occur Before The
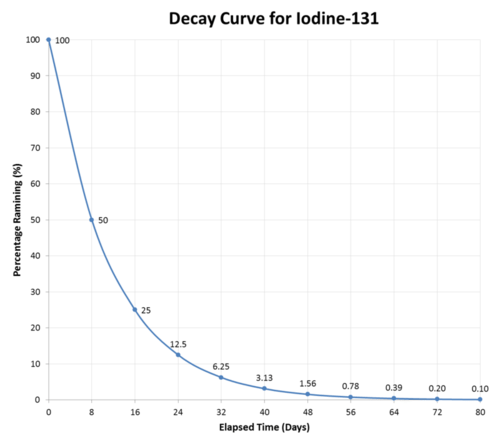


10 3 Half Life Chemistry Libretexts



Nuclear Chemistry And Radioactive Decay



Nuclear Decay Brilliant Math Science Wiki


Www Svcsd Org Cms Lib07 Ny Centricity Domain 246 Dolgos nuclear review 14 15 Pdf



Pdf Radioiodine I 131 For Diagnosing And Treatment Of Thyroid Diseases



5 3 Types Of Radiation Chemistry Libretexts
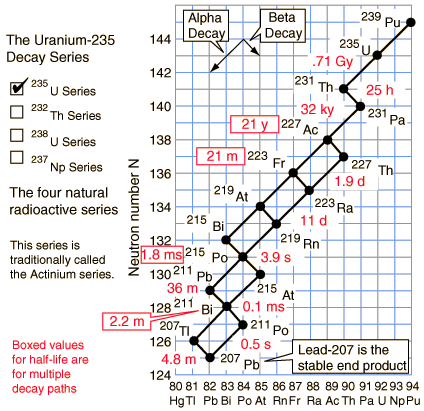


Radioactive Decay New World Encyclopedia


Nuclear Fission Fragments



3 1 Nuclear Chemistry And Radioactive Decay Chemistry Libretexts


Explaining Alpha Beta Plus Beta Minus Radioactive Emission Decay Nuclear Equations Production Artificial Radioisotopes Neutron Bombardment Positron Emission Cyclotron Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes
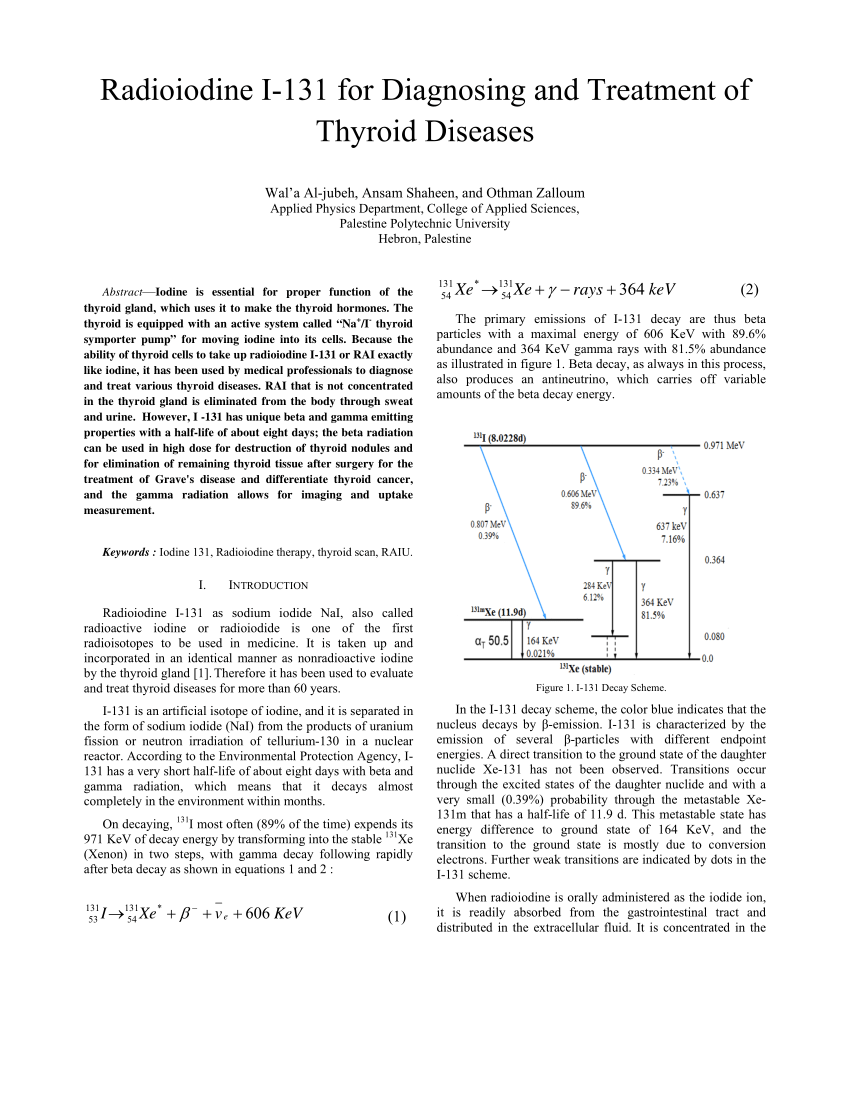


Pdf Radioiodine I 131 For Diagnosing And Treatment Of Thyroid Diseases


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf
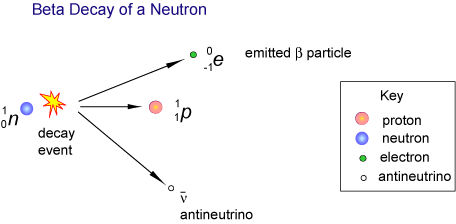


How Do You Write An Equation For The Beta Decay Of Sodium 24 Socratic



Solved Exercise 2 Write Complete Nuclear Equations For T Chegg Com


Radioactive Decay



Nuclear Chemistry And Radioactive Is The Half Life Of A Radioactive Isotope If A 500 0g Sample Decays To 62 5g In 24 3 Hours 6 What Fraction Of A Sample Of Co 60 Will Pdf Document



Iodine 131 Wikipedia



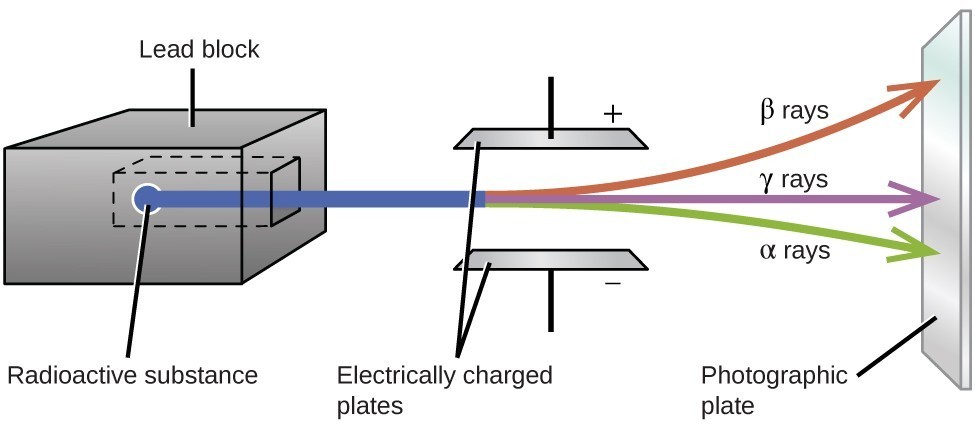
Solved The Three Types Of Natural Radioactive Decay Are Chegg Com


21 3 Radioactive Decay Chemistry


Q Tbn And9gcrnbavuxvnctctqqarsn34pzott9gvclpobgdmp2u4v4tgxcn02 Usqp Cau


Radioactive Transitions



Decay Constant Radioactivity Nuclear Power


Explaining Alpha Beta Plus Beta Minus Radioactive Emission Decay Nuclear Equations Production Artificial Radioisotopes Neutron Bombardment Positron Emission Cyclotron Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes



What Is Radioactive Decay Definition


Pplato Flap Phys 9 2 Radioactive Decay


1


Writing Natural Nuclear Decay Expressions


Iodine 131 Wikiwand



In One Form Of Beta Decay Potassium Decays To Produce Calcium What Values Do The Letters Represent Brainly Com



Nuclear Fission Product Wikipedia


Explaining Alpha Beta Plus Beta Minus Radioactive Emission Decay Nuclear Equations Production Artificial Radioisotopes Neutron Bombardment Positron Emission Cyclotron Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes



Solved 1 Tritium H 3 Undergoes Beta Decay To Produce H Chegg Com


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf


Www Svcsd Org Cms Lib07 Ny Centricity Domain 246 Dolgos nuclear review 14 15 Pdf


Www Svcsd Org Cms Lib07 Ny Centricity Domain 246 Dolgos nuclear review 14 15 Pdf



What Is Radioactive Decay Definition



14 4 Nuclear Decay And Conservation Laws Douglas College Physics 17 Winter


Radioactive Decay Nuclear Decay Processes Ppt



Radioactive Decay Nuclear Decay Processes Ppt


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf
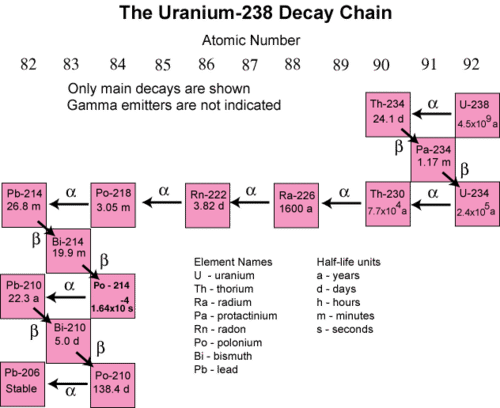


10 3 Half Life Chemistry Libretexts
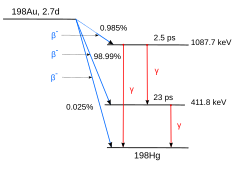


Decay Scheme Wikipedia


Radioactive Transitions


Www Pittsfordschools Org Site Handlers Filedownload Ashx Moduleinstanceid 2999 Dataid 655 Filename Unit 11 nuclear chemistry review packet key Pdf


Pplato Flap Phys 9 2 Radioactive Decay



Radioactive Decay Nuclear Decay Processes Ppt



Isotopes Of Iodine An Overview Sciencedirect Topics



Radioactive Decay Introductory Chemistry Lecture Lab


Explaining Alpha Beta Plus Beta Minus Radioactive Emission Decay Nuclear Equations Production Artificial Radioisotopes Neutron Bombardment Positron Emission Cyclotron Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes
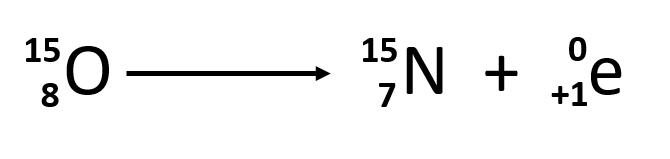


Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry


Www Svcsd Org Cms Lib07 Ny Centricity Domain 246 Dolgos nuclear review 14 15 Pdf


Www Svcsd Org Cms Lib07 Ny Centricity Domain 246 Dolgos nuclear review 14 15 Pdf
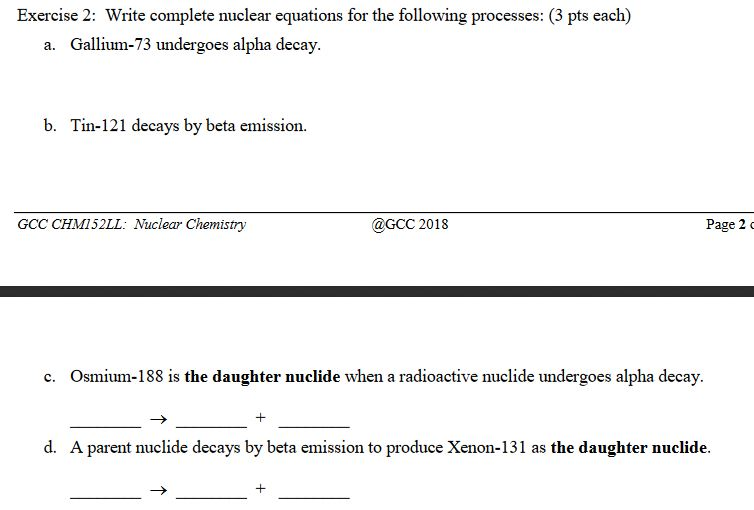


Solved Exercise 2 Write Complete Nuclear Equations For T Chegg Com


Search Q Beta Decay Example Tbm Isch
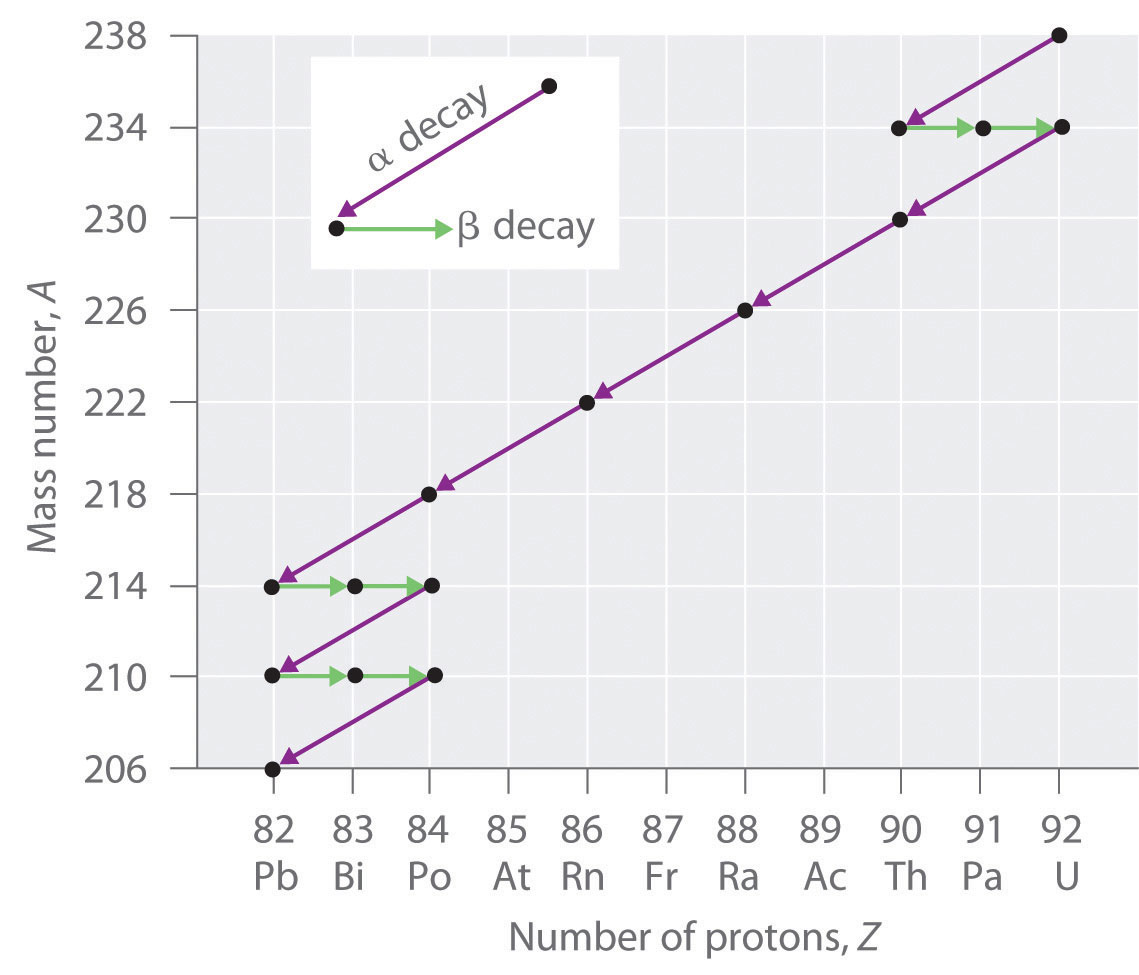


Nuclear Chemistry



Can You Write The Nuclear Decay Equation For The Beta Decay Of Iodine 131 Socratic



21 3 Radioactive Decay Chemistry
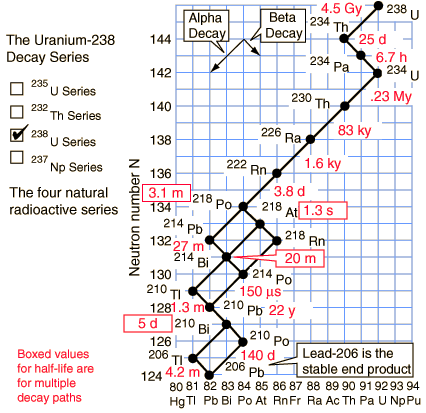


Radioactive Decay New World Encyclopedia
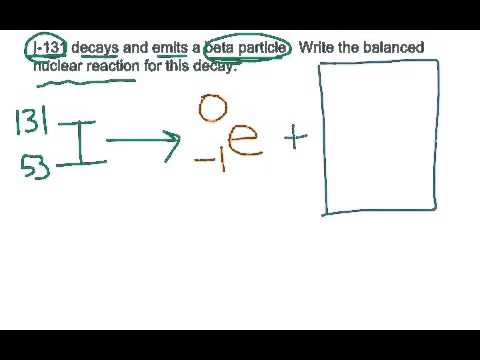


Nuclear Reaction Beta Emission By Iodine 131 Youtube
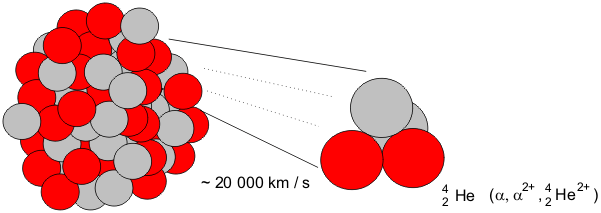


Radioactive Decay


Arxiv Org Pdf 1303 6953



Solved D Worksheet 5 Nuclear Decay Chem 163 Name Sectio Chegg Com


Www Manhassetschools Org Cms Lib Ny Centricity Domain 796 Answer key to practice in note packet Pdf



The Radioactivity Of Iodine 131 Practice Khan Academy



Ndc 698 005 Sodium Iodide I 131 Sodium Iodide I 131



Iodine 131 An Overview Sciencedirect Topics


Http Www Shscience Net Mod Resource View Php Id 4597



Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry



コメント
コメントを投稿